Soeklig op Covid-vaksiene
Hoe werk die Covid-vaksiene en hoe veilig en doeltreffend is die vaksiene?
Mari Hudson het inlligting oor die vaksiene uit betroubare bronne saamgestel.
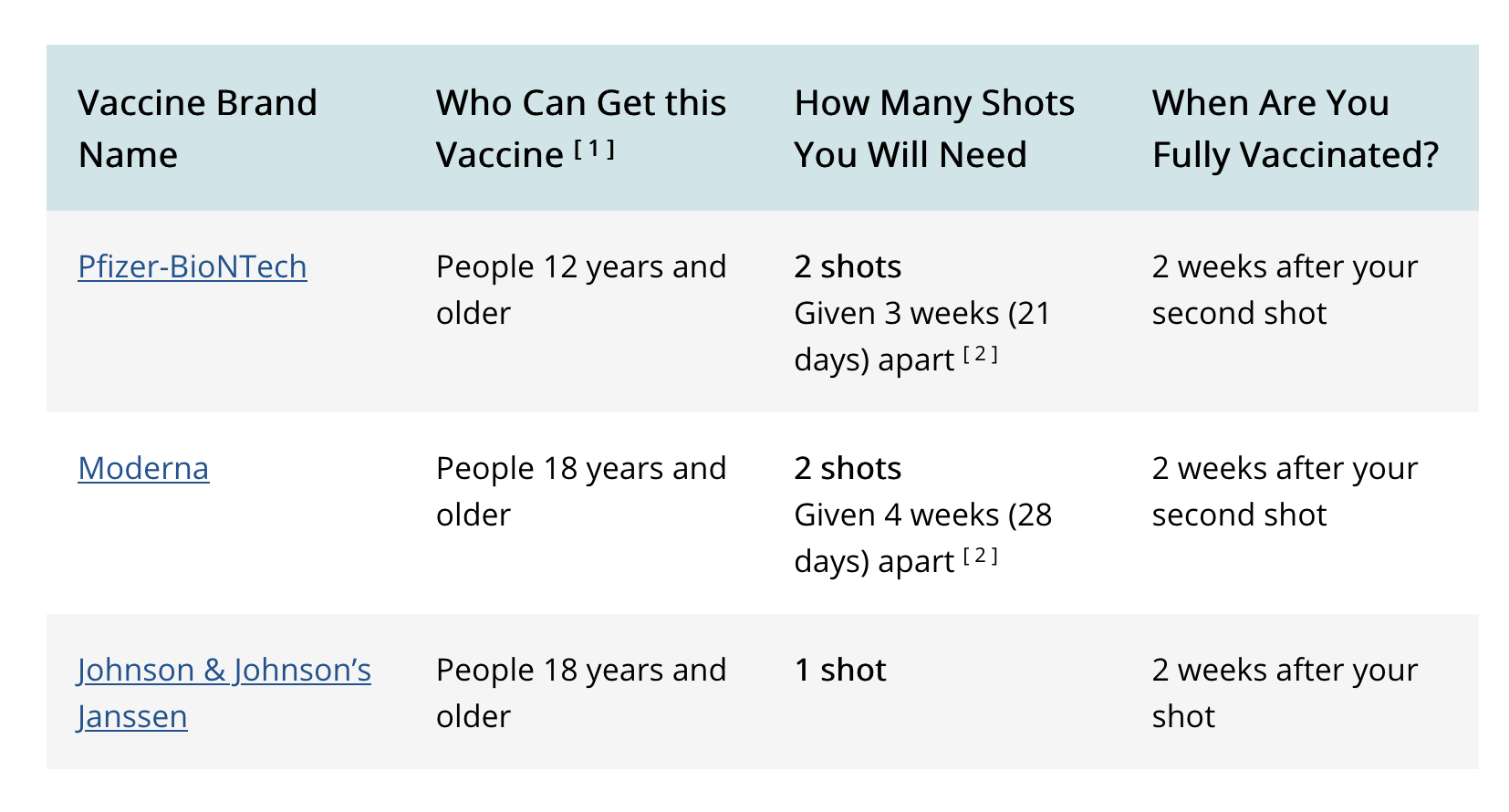
Drie vaksiene is tans deur die FDA vir gebruik in die VSA goedgekeur. In SA is J&J en Pfizer goedgekeur deur SAHPRA.
 Pfizer-BioNTech
Pfizer-BioNTech
How it works: This is a messenger RNA (mRNA) vaccine, which uses a relatively new technology. Unlike vaccines that put a weakened or inactivated disease germ into the body, the Pfizer-BioNTech mRNA vaccine delivers a tiny piece of genetic code from the SARS CoV-2 virus to host cells in the body, essentially giving those cells instructions, or blueprints, for making copies of spike proteins (the spikes you see sticking out of the coronavirus in pictures online and on TV). The spikes do the work of penetrating and infecting host cells. These proteins stimulate an immune response, producing antibodies and developing memory cells that will recognize and respond if the body is infected with the actual virus.
How well it works: 95% efficacy in preventing COVID-19 in those without prior infection. The researchers report that the vaccine was equally effective across a variety of different types of people and variables, including age, gender, race, ethnicity, and body mass index (BMI)—or presence of other medical conditions. In clinical trials, the vaccine was 100% effective at preventing severe disease. In late March, a small CDC study that enrolled 3,950 health care personnel, first responders, and other essential and frontline workers showed the vaccine to be 90% effective upon full immunization (at least 14 days after the second dose) in real-world conditions.
How well it works on virus mutations : In early May, the Pfizer-BioNTech vaccine was found to be more than 95% effective against severe disease or death from the Alpha variant (first detected in the United Kingdom) and the Beta variant (first identified in South Africa) in two studies based on real-world use of the vaccine. While the efficacy against infection varied between the two studies, both also showed the vaccine provides strong protection. As far as the Delta variant (first seen in India), two studies reported by Public Health England that have not yet been peer reviewed showed that full vaccination (after two doses) is 88% effective against symptomatic disease and 96% effective against hospitalization.
Johnson & Johnson
On February 27, 2021, the FDA granted emergency use approval for a different type of vaccine, called a carrier, or virus vector, vaccine. In early April, the CDC and FDA issued a joint recommendation for states to halt use of the Johnson & Johnson vaccine “out of an abundance of caution” during an investigation into reports of six rare, but serious clotting problems among women ages 18 to 48, occurring six to 13 days after vaccination (the recommendation fell short of an order to stop using the vaccine, leaving final decisions to the individual states).
But on Friday, April 23, the Food and Drug Administration (FDA) ended its recommended pause on the vaccine and will add a warning label about an uncommon, but potentially serious, blood clotting disorder.
In comparison to the Pfizer and Moderna vaccines, this one is easier to store (in refrigerator temperature), and requires only a single shot, which has made it easier to distribute and administer. An analysis released by the FDA in late February showed that the vaccine may reduce the spread of the virus by vaccinated people.
Status: Emergency use in the U.S.; authorized for use in the European Union (under the name Janssen).
Recommended for: Adults 18 and older.
Dosage: Single shot. In November, Johnson & Johnson announced it would launch a second Phase 3 clinical trial to study using two doses, two months apart, to see if that regimen will provide better protection.
Common side effects: Fatigue, fever headache, injection site pain, or myalgia (pain in a muscle or group of muscles), all of which generally resolve within a day or two. It has had noticeably milder side effects than the Pfizer and Moderna vaccines, according to the FDA report released in late February. No one suffered an allergic reaction in clinical trials for the vaccine, according to the company.
How it works: This is a carrier vaccine, which uses a different approach than the mRNA vaccines to instruct human cells to make the SARS CoV-2 spike protein. Scientists engineer a harmless adenovirus (a common virus that, when not inactivated, can cause colds, bronchitis, and other illnesses) as a shell to carry genetic code on the spike proteins to the cells (similar to a Trojan Horse). The shell and the code can’t make you sick, but once the code is inside the cells, the cells produce a spike protein to train the body’s immune system, which creates antibodies and memory cells to protect against an actual SARS-CoV-2 infection.
How well it works: 72% overall efficacy and 86% efficacy against severe disease in the U.S.
How well it works on virus mutations: This vaccine’s effectiveness has been shown to offer protection against the Alpha variant. According to the analyses the FDA released in late February, there was 64% overall efficacy and 82% efficacy against severe disease in South Africa, where the Beta variant was first detected. More data is needed to determine the effectiveness of the vaccine against the Delta variant.
Moderna
Moderna’s vaccine was the second one authorized for emergency use in the U.S.—it received FDA EUA on December 18, 2020, about a week after the Pfizer vaccine. Moderna is also an mRNA vaccine, using the same technology as the Pfizer-BioNTech one and with a similarly high efficacy at preventing symptomatic disease. Also, the Moderna vaccine was slightly less effective in clinical trials—about 86%—in people who are 65 and older.
Status: Emergency use in the U.S.; authorized for use in the European Union.
Recommended for: Adults 18 and older.
Dosage: Two shots, 28 days apart
Common side effects: Similar to the Pfizer vaccine, side effects can include chills, headache, pain, tiredness, and/or redness and swelling at the injection site, all of which generally resolve within a day or two. On rare occasions, mRNA vaccines have appeared to trigger anaphylaxis, a severe reaction that is treatable with epinephrine (the drug in Epipens®). For that reason, the CDC requires vaccination sites to monitor everyone for 15 minutes after their COVID-19 shot, and for 30 minutes if they have a history of severe allergies.
How it works: Similar to the Pfizer vaccine, this is an mRNA vaccine that sends the body’s cells instructions for making a spike protein that will train the immune system to recognize it. The immune system will then attack the spike protein the next time it sees one (attached to a real SARS CoV-2 virus).
How well it works: 94.1% effective at preventing symptomatic infection in people with no evidence of previous COVID-19 infection. The vaccine appeared to have high efficacy in clinical trials among people of diverse age, sex, race, and ethnicity categories and among persons with underlying medical conditions (although as mentioned above, the efficacy rate drops to 86.4% for people ages 65 and older). In late March, a small CDC study that enrolled 3,950 health care personnel, first responders, and other essential and frontline workers showed the vaccine to be 90% effective upon full immunization (at least 14 days after the second dose) in real-world conditions.
How well it works on virus mutations: Some research has suggested that Moderna’s vaccine may provide protection against the Alpha and Beta variants. Researchers are still studying this. While more research is needed on Moderna’s efficacy against Delta, some experts believe it may work similarly to Pfizer since both are mRNA vaccines.
Oxford-AstraZeneca
This vaccine, which is currently being distributed in the United Kingdom and other countries, is distinguished from some of its competitors by its lower cost—it’s cheaper to make per dose, and it can be stored, transported, and handled in normal refrigeration for at least six months. Some countries temporarily suspended use of this vaccine in March after a small number of recipients developed blood clots. In April, a European Medicines Agency (EMA) safety committee concluded “unusual blood clots with low blood platelets should be listed as very rare side effects” that could occur within two weeks of receiving the vaccine. While the U.K. called for further investigation, EMA regulators stressed that the benefits of the vaccine still outweigh the risks.
Status: Not available in the U.S.,authorized for use in the European Union (under the name Vaxzevria).
Recommended for: Adults 18 and older
Dosage: Two doses, four to 12 weeks apart
Common side effects: Tenderness, pain, warmth, redness, itching, swelling or bruising at the injection site, all of which generally resolve within a day or two.
How it works: Similar to the Johnson & Johnson’s vaccine, this is a carrier vaccine, made from a modified version of a harmless adenovirus. The final product contains the spike protein found in SARS-CoV-2. When that protein reaches the body’s cells, the immune system mounts a defense, creating antibodies and memory cells to protect against an actual SARS-Cov2 infection.
How well it works: AstraZeneca updated its data analysis of its phase 3 trials in March, showing its vaccine to be 76% effective at reducing the risk of symptomatic disease 15 days or more after receiving the two doses, and 100% against severe disease. The company also said the vaccine was 85% effective in preventing COVID-19 in people over 65. The company’s update came a few days after the National Institute for Allergy and Infectious Diseases (NIAID) expressed concern over new data AstraZeneca had submitted in advance of requesting an EUA from the FDA. The NIAID said that data may have included outdated information, which would make its efficacy data incomplete.
How well it works on virus mutations: So far it seems to work better against Alpha variant than the Beta variant. A paper in early February (not yet peer-reviewed) cited 74.6% efficacy against the Alpha variant. However, the vaccine did not protect as well against mild and moderate cases in people infected with the Beta variant. Therefore, South Africa halted its rollout while scientists continue to study whether the vaccine can prevent severe illness and death in people infected with this variant. As far as the Delta variant, two recent studies (neither has been peer-reviewed) showed, respectively, that full vaccination after two doses is 60% effective against symptomatic disease and 93% effective against hospitalization.
Novavax
This vaccine has been shown to be effective not only against COVID-19, but also against the mutations that have emerged in Great Britain and, to a lesser extent, South Africa. While the other breakthrough vaccines have been either mRNA or vector platforms, the Novavax vaccine is yet another type, called a protein adjuvant. It is also simpler to make and can be stored in a refrigerator.
Status: Still completing clinical trials
Recommended for: The vaccine is being studied in adults ages 18-84
Dosage: 2 doses, three weeks apart
Common side effects: Injection site tenderness, fatigue, headache, muscle pain.
How it works: Unlike the mRNA and vector vaccines, this is a protein adjuvant (an adjuvant is an ingredient used to strengthen the immune response). While other vaccines trick the body’s cells into creating parts of the virus that can trigger the immune system, the Novavax vaccine takes a different approach. It contains the spike protein of the coronavirus itself, but formulated as a nanoparticle, which cannot cause disease. When the vaccine is injected, this stimulates the immune system to produce antibodies and T-cell immune responses.
How well it works: 90% effective against lab-confirmed, symptomatic infection and 100% against moderate and severe disease in Phase 3 trial results released in a company statement in June. The company says the vaccine was 91% protective of people in high-risk populations such as people older than 65, those with health conditions that increase risk of complication, and those in situations where they are frequently exposed to the virus.
The company says the vaccine was 91% protective of people in high-risk populations such as people older than 65, those with health conditions that increase risk of complication, and those in situations where they are frequently exposed to the virus.
How well it works on virus mutations: Novavax says the vaccine is 93% effective against “predominantly circulating variants of concern and variants of interest,” and 100% against variants “not considered variants of concern/interest.” (It’s important to note that the study was conducted in the U.S. and Mexico, when Alpha was the predominant strain in the U.S., although other variants were on the rise.) More data is needed to determine the effectiveness of Novavax against the Delta variant.
Editor’s Note: This article was reviewed by Yale Medicine infectious diseases specialist Jaimie Meyer, MD, MS.
Lees meer oor Covid-vaksiene:
https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines.html
https://www.yalemedicine.org/news/covid-19-vaccine-comparison
https://www.thelancet.com/journals/lanmic/article/PIIS2666-5247(21)00069-0/fulltext