J&J vaksien-onttrekking: Die perspektief en die toekoms
Die FDA het pas die vaksinering met die J&J vaksien in Amerika tydelik gestaak, en Suid-Afrika ook. Dit volg nadat 6 vroue ná vaksinering van 6,8 miljoen mense bloedstolsels ontwikkel het. Dit is 1 uit 1 miljoen, oftewel 0,0001%
Mari Hudson gesels met Prof Barry Jacobson, hematoloog van Wits en NHLS, oor die vaksien-onttrekking en die risiko vir stolselvorming tydens Covid en ná vaksinering.
Wat beteken dit alles en wat nou?
Luister om 11:30 na Gesondheid op RSG.
Uittreksel uit die verklaring wat deur die FDA en die CDC uitgereik is
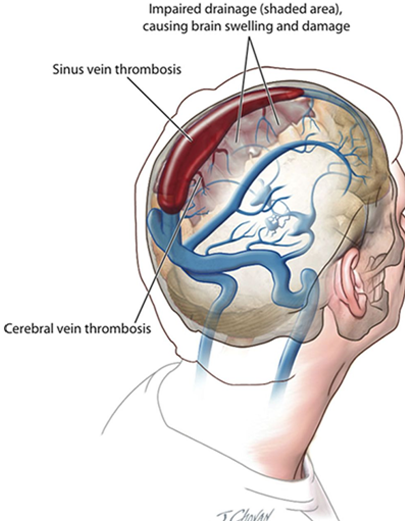
As of April 12, more than 6.8 million doses of the Johnson & Johnson (Janssen ) vaccine have been administered in the U.S. CDC and FDA are reviewing data involving six reported U.S. cases of a rare and severe type of blood clot in individuals after receiving the J&J vaccine. In these cases, a type of blood clot called cerebral venous sinus thrombosis (CVST) was seen in combination with low levels of blood platelets (thrombocytopenia). All six cases occurred among women between the ages of 18 and 48, and symptoms occurred 6 to 13 days after vaccination. Treatment of this specific type of blood clot is different from the treatment that might typically be administered. Usually, an anticoagulant drug called heparin is used to treat blood clots. In this setting, administration of heparin may be dangerous, and alternative treatments need to be given.
Die volle verklaring wat deur die FDA en die CDC uitgereik is: https://www.cdc.gov/media/releases/2021/s0413-JJ-vaccine.html
Dr Zweli Mkhize se verklaring:
We have noted the decision taken by the Food and Drug Administration in the United States of America to advise the temporary suspension of the Johnson and Johnson vaccine rollout in the US.
This has occurred to due reports of 6 females who developed unusual blood clots with low platelets. These incidents occurred between 6 and 13 days after vaccination in women between the ages of 18 and 48 years old. It must be noted that over 6.6 million citizens have been inoculated with Johnson and Johnson vaccine in the US.
In South Africa, we have not had any reports of clots that have formed after vaccination, and this is after having inoculated 289,787 health care workers under the Sisonke Protocol.
Having said that, after this advisory came to my attention I held urgent consultations with our scientists, who have advised that we cannot take the decision made by the FDA lightly. Based on their advice, we have determined to voluntarily suspend our rollout until the causal relationship between the development of clots and the Johnson and Johnson vaccine is sufficiently interrogated.
SAHPRA will collate information from Johnson and Johnson, the FDA and other regulatory bodies to make a thorough assessment of the situation and advise us as a regulatory body that has exercised its authoritative powers on the approval of the vaccine in their own right.
I humbly call for calm and patience as we ensure that we continue to be properly guided by science in ensuring the safety of our people as we rollout the vaccine campaign.
We hope that the deliberations will only take a few days. Given the preliminary literature on hand, our scientists are confident that the FDA’s decision is on a precautionary basis and we expect that this will not result in the complete withdrawal of the Johnson and Johnson vaccine from the vaccination armament.
We also wish to assure citizens that these kinds of reports are expected to emerge as part of a robust post market surveillance system- this should provide comfort that medical authorities keep a vigilant watch on all new products that are deployed into the market to ensure they remain safe and effective for human consumption. It is for this reason that we implemented the Sisonke Protocol and will also implement a similar post market surveillance study for Pfizer when we roll out the first batch of doses to health care workers.
I am glad to say, however, that there are good news in the midst of this development.
I am happy to announce that we have successfully negotiated for another 10 million doses from Pfizer and, of these, we expect just under 2 million to be delivered in May. This therefore means we have secured 30 million doses of Pfizer vaccine for this financial year.
This also reassures us that, in the extremely unlikely event that Johnson and Johnson rollout is completely halted, we will not have any impediment to proceed with phase two of the rollout with Pfizer.
We are confident that the rollout of Johnson and Johnson will resume and so, with 30 million doses of Johnson and Johnson and 30 million doses of Pfizer secured we now have enough doses to exceed the 40 million we were targeting this year. This is in line with our commitment to vaccinate as many people as possible in this financial year- in the ideal scenario we would vaccinate every single adult found in South Africa.
This development is an example of how we, as government, make commitments and work to the best of our ability to honour those commitments, however science must be respected at all times, and this sometimes means a disruption in our plans. Although we are operating in a dynamic and ever changing environment, the government is constantly seeking to secure vaccines that will protect us from the 501Y.V2 variant.
We therefore urge South Africans to be supportive of our experts and scientists as they work under enormous pressure to give us answers on this worrying development so that we may proceed safely with the global vaccination programme. We commit to keeping the public informed of all developments
Meer oor die effektiwiteit van die vaksiene
In the J&J trial, the placebo group had 16 hospitalizations and seven deaths from COVID-19, whereas the vaccine group had none, which means the vaccine provided 100 percent efficacy against hospitalizations and deaths.
For severe disease, which includes people who were sick enough with COVID-19 to require medical intervention but recovered without hospitalization, the efficacy was about 85 percent across the board in Brazil, South Africa and the U.S.
Including mild and moderate disease, the overall efficacy was 66 percent, but varied across the regions: 72 percent in the U.S., 64 percent in South Africa, and 61 percent in Brazil. “Mild and moderate outcomes” could include a range of illness, said Gandhi, and we won’t know the details until the full trial results are published, but we do know that everyone recovered without medical intervention.